
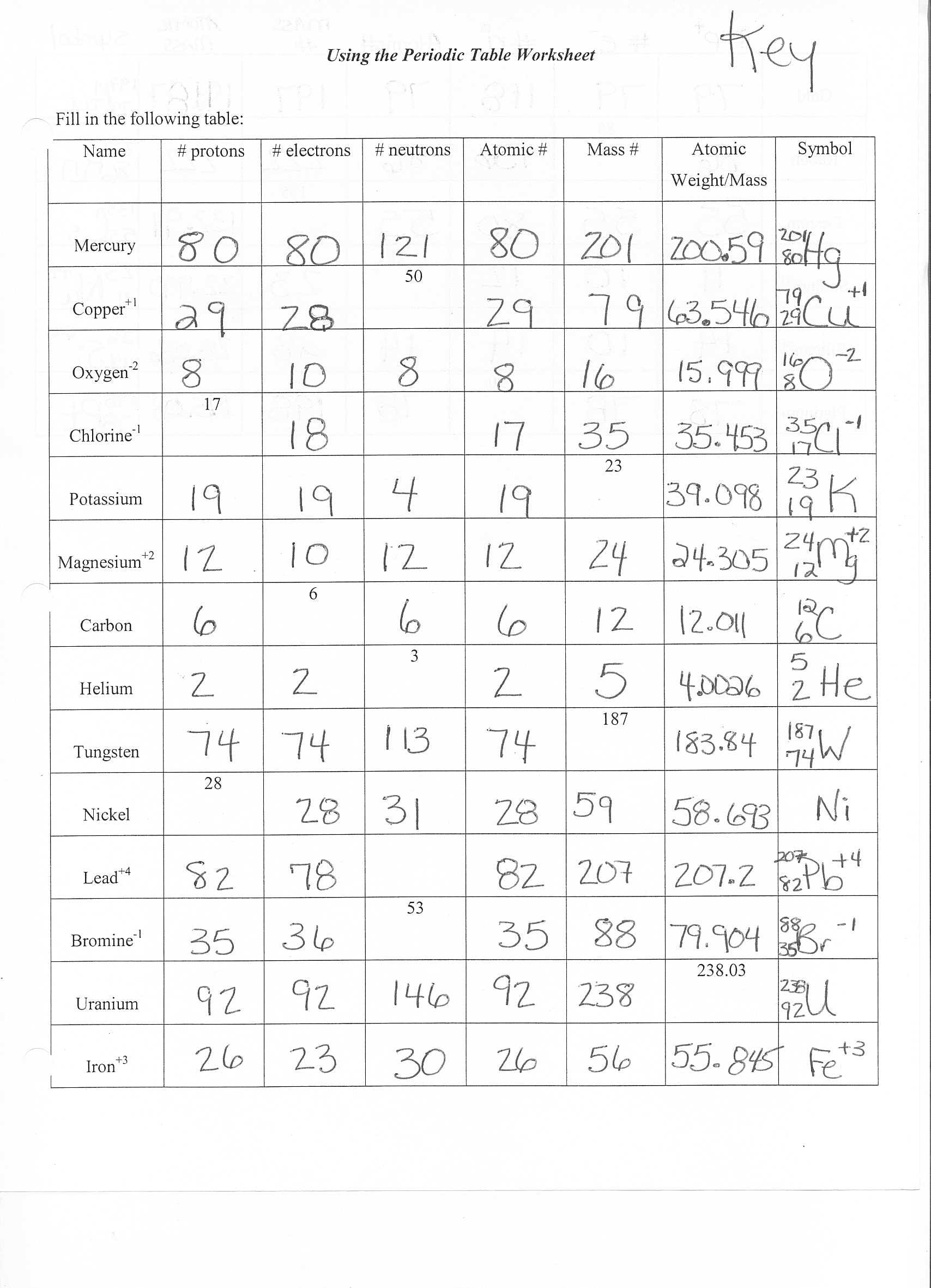
Basically, quantum numbers represent number values in the quantum system in the form of four quantum numbers. In quantum physics and chemistry, quantum numbers play a major role in denoting the locality and energy values of an electron in its atomic orbital. Let us learn what Quantum Numbers and Spin values of an electron are!ĭownload The Denotation of Quantum Numbers
To put it simply, every individual electron encompasses of four quantum numbers and two electrons must exhibit opposite spins when located in the same orbital. Pauli Exclusion PrincipleĪccording to Pauli Exclusion Principle, two or more electrons of a single atom cannot occupy the same quantum state and possess the same quantum values. Atomic spectra is nothing but a theory that represents the ground state of an atom using open electronic shells. However, Hund’s rule strictly follows the theory of atomic spectra. And then they can start double occupying of orbitals of subshell. Hund’s rule denotes that electrons must occupy every single orbital of a subshell with at least one electron with same spin direction. And hence the electronic configuration of bromine atom is 1s 22s 22p 63s 23p 64s 23d 104p 5, satisfying Aufbau principle. Two electrons out of 7 valence electrons occupy 4s orbital first and the rest occupy 4p orbital. It has 35 electrons and among which 7 electrons are valence electrons.

This is because the electrons in 3d orbital repel strongly as they are very close to the nucleus of the atom.Ĭonsider Bromine element located in the Group VII, Period 4 of the periodic table. Note to remember: The electrons prefer to occupy the lowest orbital, 4s first rather than the still lowest 3d orbital, against the rule. For ex: 7s, 5f, 6d and 7p subshells will not be filled up without the occupation of electrons in 1s to 6p subshells. With reference to the above order of occupation, it is clear that electrons will not occupy the highest energy orbitals until they already filled up the lowest energy orbitals. Have a look at the order of electron occupying energy states in its atomic orbitals:ġs<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d<7p That means, they occupy the lowest energy state in the beginning and continue to the next highest energy level and go on… Let us see how and in what are those rules: Aufbau PrincipleĪufbau is a German term and it says ‘Building Up’! Well, the principle of Aufbau denotes that electrons occupy energy states in the increasing order form. These theorems include Aufbau Principle, Hund’s Rule, and Pauli Exclusion Principle-which forms the set of general rules to write electronic configuration for any element in the periodic table. To determine the electronic configuration of an element, one must follow three important principles from quantum mechanics.
#Atomic structure practice worksheet answers plus#
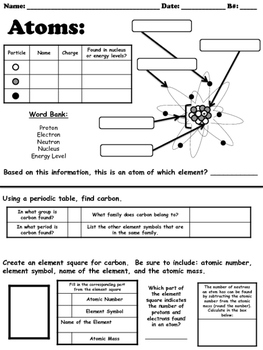
These four atomic orbitals are present around the nucleus of an atom and represent different energy states.īy studying these atomic orbitals, scientists calculate and write the location and energy state of an electron plus its interaction in the atom to create chemical bonding. Well, atomic orbitals are nothing but the energy quantum states that tell the uncertain behavior and exact location of an electron in the electron cloud. s, p, d, and f represent the four different atomic orbitals. The letters in the electronic configuration of any element i.e. For Example: The electronic configuration of Potassium is 1s 22s 22p 63s 23p 64s 1 Well, using the periodic table, anyone can easily write the electronic configuration of any element. This notation also helps in understanding the bonding capacity of electrons in an atom through magnetic and other chemical features. The electron configuration of an element is a standard representation of its electron arrangement in the orbitals of its atom. Definition and Basics of Electronic Configuration Let us learn more about the electronic configuration along with some awesome worksheets and orbital diagrams in this article.


 0 kommentar(er)
0 kommentar(er)
